Description
| Manufacturer # | 49348007002 |
| Brand | sunmark‚® |
| Manufacturer | McKesson Brand |
| Active Ingredients | Dimenhydrinate |
| Application | Nausea Relief |
| Dosage Form | Tablet |
| Generic Drug Code | 18231 |
| NDC Number | 49348-0070-02 |
| Strength | 50 mg Strength |
| UNSPSC Code | 51171800 |
| Volume | 12 per Box |
| Latex Free Indicator | Not Made with Natural Rubber Latex |
Features
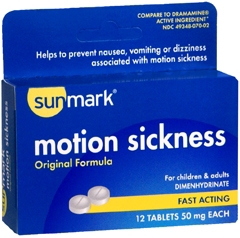
- sunmark‚® Motion Sickness Tablets
- 50 mg
- For prevention and treatment of these symptoms associated with motion sickness: nausea, vomiting, dizziness. Fast acting.
- For children and adults.
- Compares to Dramamine‚®.
- Not Made With Natural Rubber Latex.
- Packaged: 12 Per Box
- Dramamine is a registered trademark of Pfizer Inc.
NDC: 49348007002